Products
Prima RNApols™
Wondering which RNApol is right for you?
| Product | YIELD^ | Reduced dsRNA^^ | CAPPING | Nucleotides (Modified & Unmodified) |
T7 Promoter Compatibility |
|||
|---|---|---|---|---|---|---|---|---|
| Linear <5 kb | Linear >5 kb & saRNA | AG Cap Analog | AU Cap Analog | Enzymatic Capping |
||||
|
Prima ExTend Available Now |
✓ | ✓✓✓ | ✓✓✓ | ✓ | ✓ | ✓ | ✓ | No |
|
Prima ExTend Cap AU Available Now |
✓ | ✓✓✓ | ✓✓✓ | ✓ | ✓✓✓ | ✓ | ✓ | No |
|
Prima Cap AG 2H 2026 Release |
✓✓✓ | ✓ | ✓✓✓ | ✓✓✓ | ✓ | ✓ | ✓ | No |
|
Prima Drop-in 2H 2026 Release |
✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓ | ✓ | Yes |
✓✓✓ Substantive Improvement over T7
✓✓ Improvement over T7
✓ Greater than or equal to performance with T7
^ Yield of target mRNA per unit of IVT input (DNA template, Cap Analog, etc)
^^ & Other IVT by-products, abortive transcripts, etc
Source Our Prima RNApols
By outperforming the industry-standard T7 RNA polymerase, our Prima RNApols improve the cost and quality profiles of pharmaceutical mRNA manufacturing across formats (linear, self-amplifying, circular) and a range of DNA template sequences and lengths, while remaining compatible with various capping chemistries, modified nucleotides, and IVT conditions.
Prima ExTend Cap AU Kit

Contains:
20,000 U RNA Polymerase (100 µL)
9.5 kb Linearized saRNA Template (25 µL)
5x Reaction Buffer (100 µL), 4 vials
For Research Use Only
Prima ExTend Kit
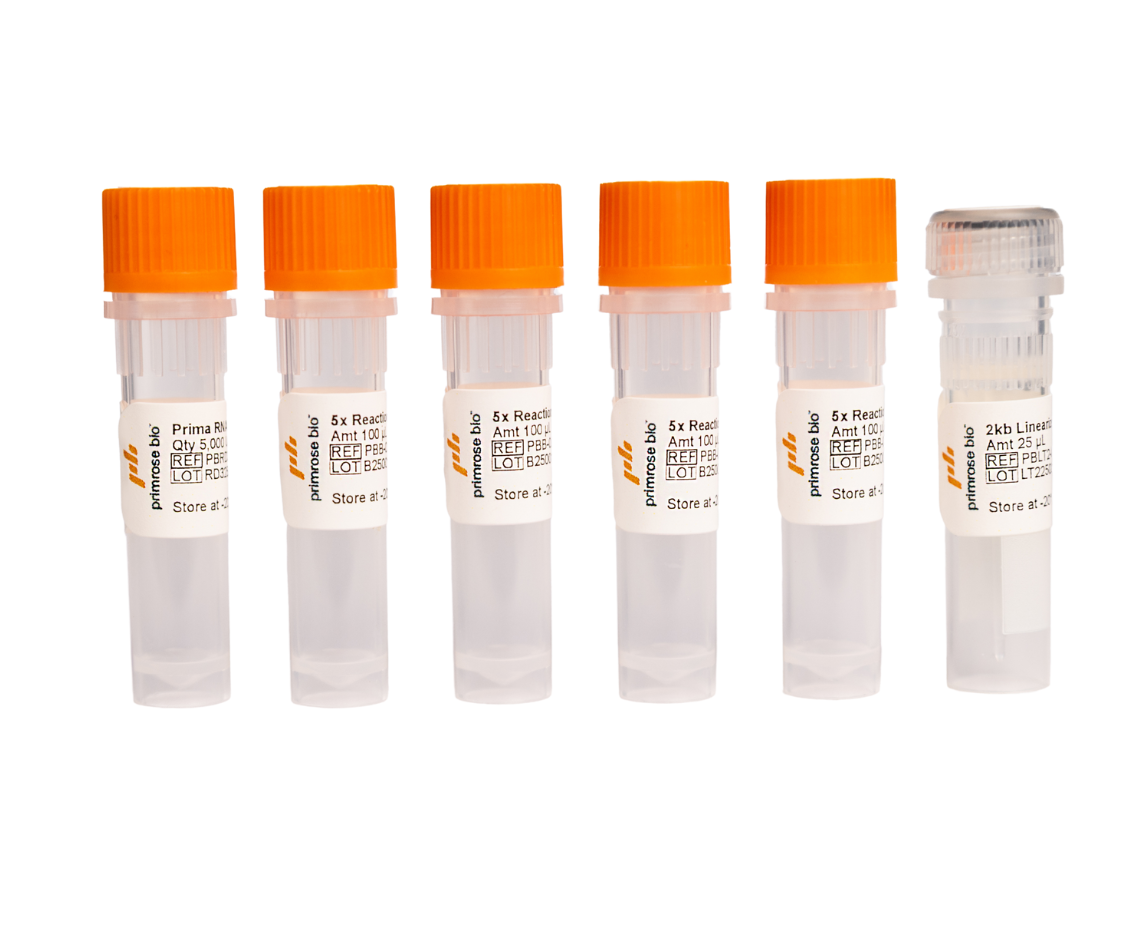
Contains the following for 50 IVT reactions:
20,000 U RNA Polymerase (100 µL)
4, 5x Reaction Buffer (100 µL)
2 kb Linearized DNA Template (25 µL)
For Research Use Only
Prima ExTend GMP
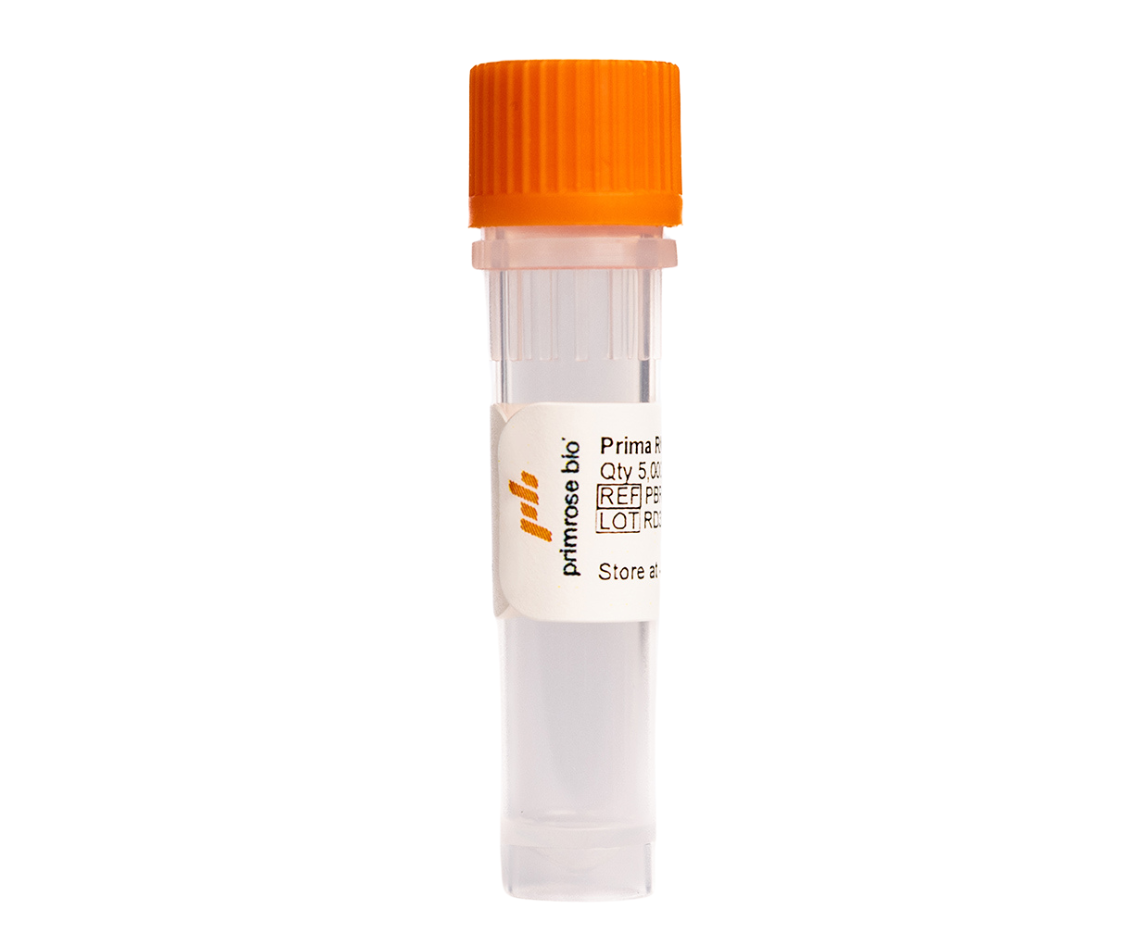
Contains 20,000 U RNA Polymerase
(100 µL)
GMP-grade Prima RNApols are manufactured and released under ISO 13485:2016 certified quality systems.
Prima ExTend GMP – Large
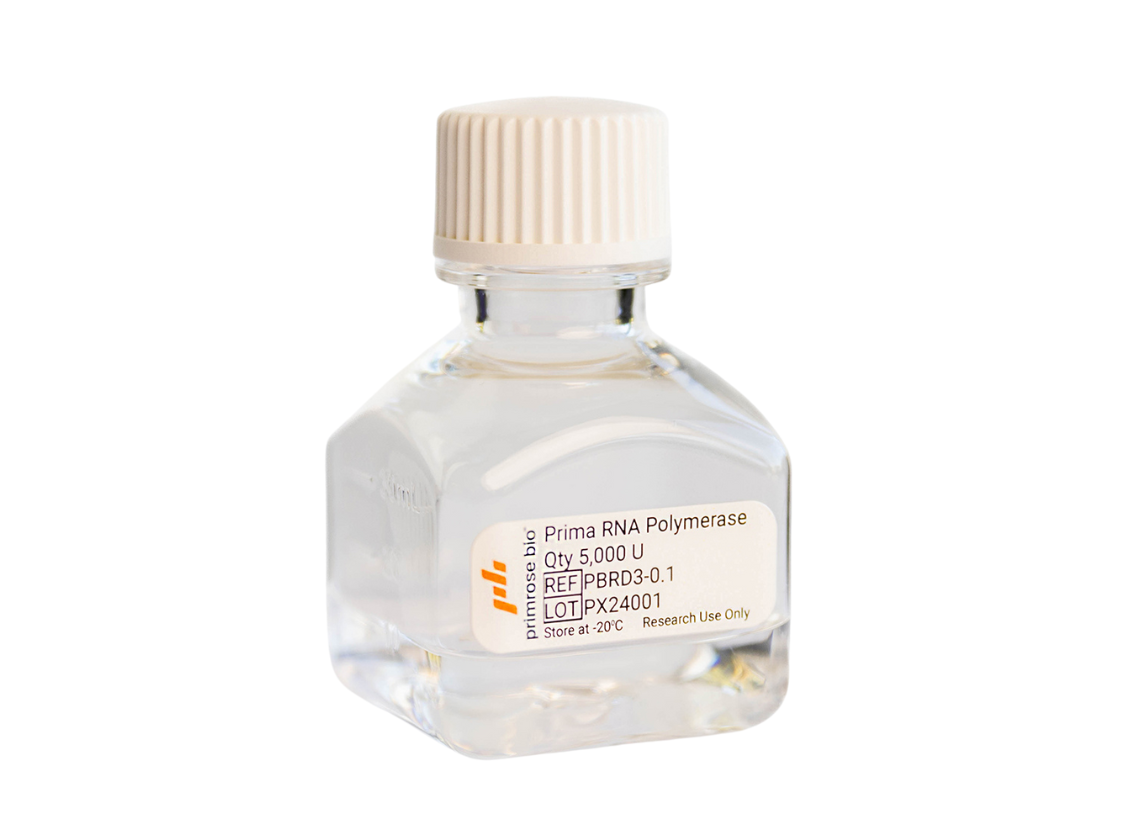
Contains 2,000,000 U RNA Polymerase
(10 mL)
GMP-grade Prima RNApols are manufactured and released under ISO 13485:2016 certified quality systems.
